ABfinity SNAP25 and VAMP1 Antibodies for Studying Neuronal Synaptic Vesicle Exocytosis
Introduction
Download the PDFThe functional unit of the nervous system, the terminally differentiated neuron, is an exquisitely specialized cell with an extraordinary structure that displays a high degree of polarity. From the neuronal cell body arise branching dendrites that receive signals from other neurons and an extended axon that transmits signals to other cells [1]. The functional connections between neurons are governed by specialized structures known as synapses, where membrane-bound synaptic vesicles carry and release neurotransmitters. Hundreds of proteins regulate the proper release of neurotransmitters from these synaptic vesicles. Synaptic plasticity is involved in memory and learning, and improper synaptic function is implicated in a myriad of disorders, including autism, schizophrenia, Alzheimer’s disease, and numerous other neuropsychiatric disorders [2]. Elucidating the biochemical basis of synaptic diversity, composition, and function are therefore fundamental to our understanding and treatment of these disorders.
Soluble NSF attachment protein receptor (SNARE) proteins belong to a multi-member protein family that plays an important role in the release of neurotransmitters through synaptic vesicle fusion and exocytosis.
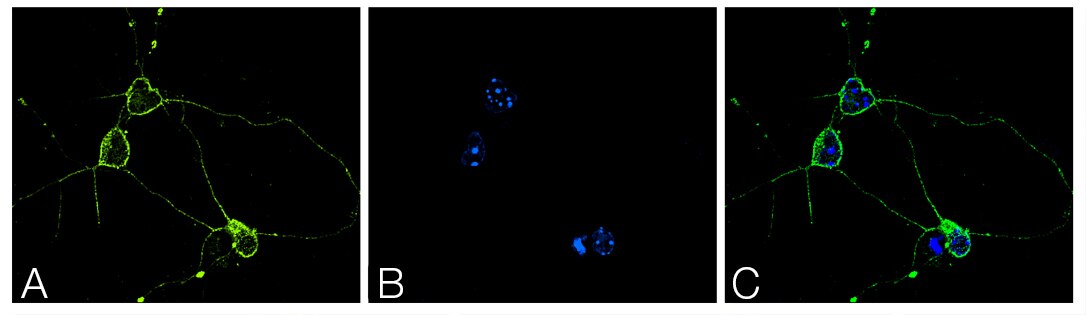
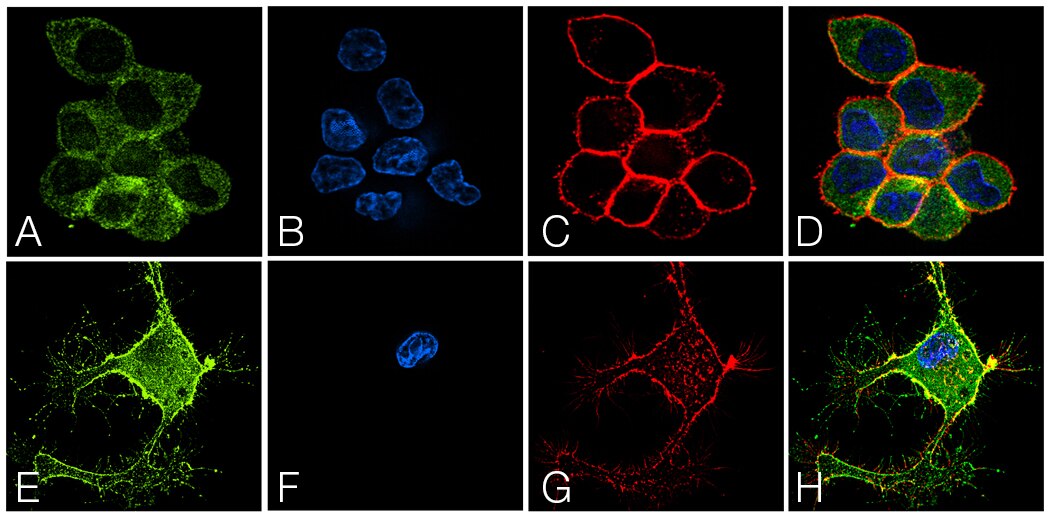
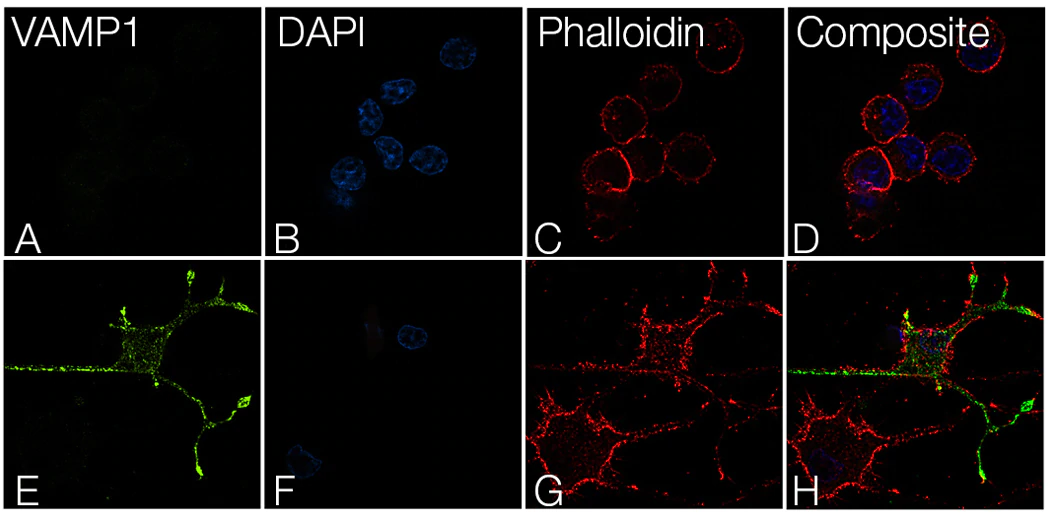
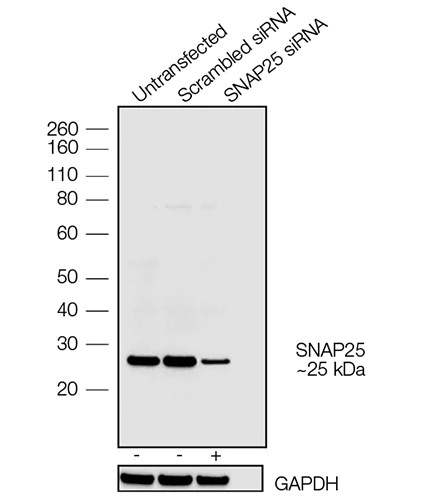
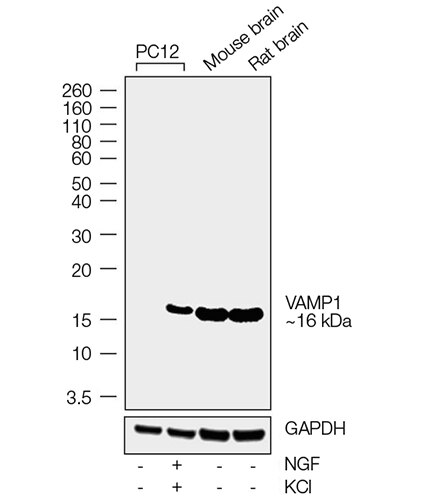
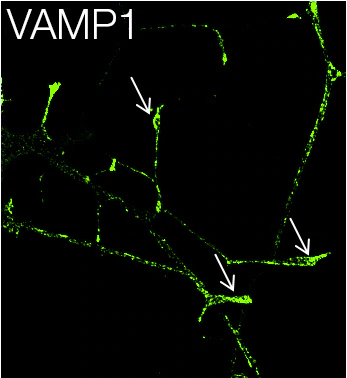
A synaptosomal-associated protein of 25 kDa (SNAP25) and vesicle-associated membrane protein-1 (VAMP1), also known as synaptobrevin (Syb), are the two major SNARE proteins that mediate synaptic vesicle exocytosis [3,4]. The therapeutic relevance of these proteins is underscored by the effects of certain mutations in disease outcomes. A dominant negative mutation in SNAP25 leads to impaired vesicle trafficking in a mouse model [5], and other mutations are a novel cause of epilepsy and genetic disability [6]. VAMP1 mutations have been reported in congenital myasthenic syndrome and hereditary spastic ataxia [7,8].
In neurons, SNAP25 primarily resides in axons and nerve endings, but also displays significant localization in the cytoplasm, and in the plasma membrane through its palmitoylated cysteine residues [9]. Treatment with nerve growth factor (NGF) enhances expression of SNAP25 1.8–3 fold in PC12 cells [10]. VAMP1 is an integral membrane protein present in synaptic vesicles. VAMP1 and synaptic protein syntaxin interact with SNAP25 after the latter forms a binary complex with another protein, tagmin. This interaction plays a critical role in fusion of the synaptic vesicle to the presynaptic membrane [11].